Ozone is a molecule made up of three oxygen atoms, often referenced as O3. Ozone is formed when heat and sunlight cause chemical reactions between oxides of nitrogen (NOX ) and Volatile Organic Compounds (VOC), which are also known as Hydrocarbons. This reaction can occur both near the ground and high in the atmosphere.
Just remember that ozone is "Good Up High, Bad Nearby."
Stratospheric ozone is good ozone . It forms about 10-30 miles above the Earth's surface and forms a protective layer, called the ozone layer, that shields us from too much of the sun's harmful ultraviolet radiation (UV).
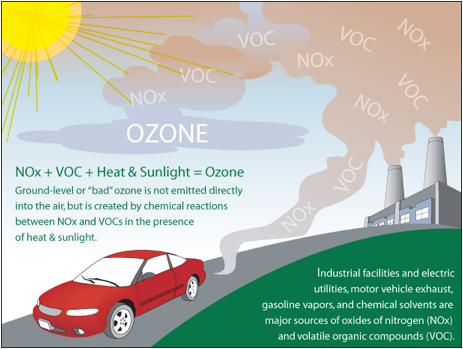 Ground-level ozone is bad ozone . Ozone harms human health and the environment when it forms close to the ground. The most significant things that cause ground-level ozone to form are:
Ground-level ozone is bad ozone . Ozone harms human health and the environment when it forms close to the ground. The most significant things that cause ground-level ozone to form are:
- NOX and VOCs (from mobile source emissions and industrial processes ), and
- UV radiation (from sunlight).
We see higher ground-level ozone amounts most often in summer, due to increased amounts of UV radiation during the longer days, but ozone can still form in spring, fall, and even winter given the right conditions.
Even though the emission sources that contribute to ground-level ozone are typically found in urban areas, strong winds can also move it into rural areas, causing them to have high amounts of ground-level ozone.
People with respiratory conditions such as asthma, or those who are active outside on days when ozone amounts are high can feel shortness of breath, wheezing, and coughing. We can all take actions to help protect ourselves and reduce the damaging effects of this pollutant on our health and environment.
Contact
Mary Peyton Wall, Manager, (803) 898-4064